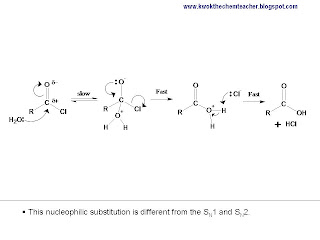
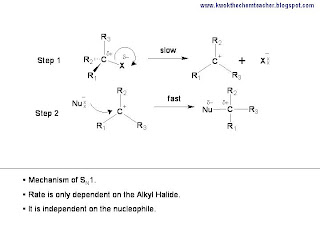
When an alkyl halide reacts with a nucleophile in a nucleophilic substitution, it has two possible pathway: SN1 or SN2. The former is a two steps mechanism, where an intermediate is formed. While the latter is a single step reaction, with no intermediate formed.
Hence, a simple tool to predict whether an alkyl halide reacts in a
SN1 or
SN2 manner, it is dependent on whether an carbocation intermediate can be formed. If the carbocation intermediate can be formed, it must be because it is stable. In an earlier article, we discussed about carbocation and you can read it
here.
This entry intends to elaborate further about the possible types of carbocations that can be formed from different alkyl halides. The following illustration gives us some examples of such alkyl halides which undergoes SN1 mechanism.
The tertiary alkyl halide tends to undergo SN1 mechanism because it is able to form a tertiary carbocation (as shown below) which is made stable by the 3 alkyl groups it is attached to. The alkyl groups being electron donating, allow the positive charge to be delocalised by induction. The fewer the alkyl groups, the less stable the carbocation would be and hence the less likely for the alkyl halide to undergo SN1.
However, there are other alkyl halides which are not tertiary and they are able to undergo SN1. This is because they have other portion(s) of the ion which enables the positive charge to be delocalised and hence making the ion stable.
In the following two cases (carbon with positive charge is attached to a C=C and benzene ring), the carbocation are stable because the positive charge is being delocalised by resonance. This happens when the p orbital of the carbon with the positive charge and the p orbital of the carbon that makes the alkene (or benzene) can overlap with each other.
The overlapping of all the relevant p orbital enables the pi electrons are able to flow towards the carbon with the positive charge, hence the flow of pi electrons cause the positive charge to be delocalised; This form of delocalisation is delocalisation by resonance. Therefore, it is because these two carbocations are made stable, their correspond alkyl halide would react with a nucleophile in a SN1 manner.
As delocalisation of the positive charge into the benzene ring is quite extensive, if the carbon with the positive charge is attached to a cyclohexene (as shown below). The stabilising effect from benzene will make the carbocation more stable than the latter's case.
-- -- -- -- --
Article written by Kwok YL 2010.
Disclaimer and remarks:
- If you would like to use this source, kindly drop me a note by leaving behind a comment with your name and institution. I am all for sharing as the materials on this blog is actually meant for the education purpose of my students.
- This material is entirely written by the author and my sincere thanks will be given to anyone who is kind, generous and gracious to point out any errors.