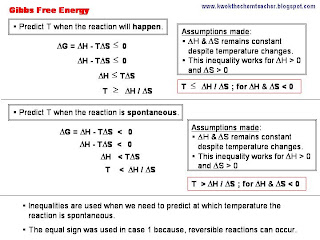
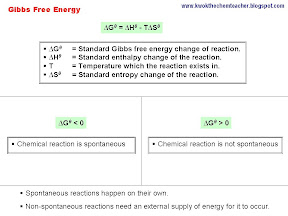
In this entry, I will be elaborating about the different definitions of enthalpy changes. In each set, there is an illustration and a subsequent set of annotations to give further elaboration.
(A) First set:

(1) In simple terms, the enthalpy change of reaction is per mole of reaction. What this implies is that the thermochemical equation is balanced and all the stoichiometric coefficients are intergers.
(2) In the
enthalpy change of formation, do note that the non-metal substances which exist as simple discrete molecules such as hydrogen and oxygen, exist as H
2 and O
2 respectively.
(3) As combustion are exothermic reactions, we will expect all enthalpy change of combustion to be exothermic.
(B) Second set:

(4) The
enthalpy change of atomisation stresses on the formation of one mole of gaseous atoms. Please do not be confused with this definition and that of enthalpy change of formation.
(5) The second ionisation energy simply refers to the second electrons removed.
(6) While the second electron affinity refers to the second electron added.
(7)
Lattice energy describes the strength of an ionic bond. It is directly proportional to the product of the charges of the cation and the anion and it is inversely proportional to the sum of the respective radius of the cation and anion. As lattice energy talk about formation of the ionic bond only, it is an exothermic process.
(C) Third set:
(8)
Enthalpy change of solution is the enthalpy change when one mole of substance dissolves in water. We cannot assume that dissolving substance into water does not produce or require heat.
(9)
Enthalpy change of neutralisation stresses on the formation of one mole of water in a reaction between an acid and a base. Between a strong acid and a strong base, the enthalpy change of neutralisation is -57 kJ mol
-1.
However, if a weak acid and a strong base is used (or vice versa), the enthalpy change of neutralisation is less exothermic than -57 kJ mol
-1. This is because some amount of heat is required to dissociate the weak acid (or weak base) completely.
(10)
Bond energy - do take note that it is defined to be the breaking of one mole of a given covalent bond to give gaseous products.
Hence, in order to ensure it is the energy that breaks the covalent bond, the substance must exist as a gas first. Generally, substances that have covalent bonds exist as simple discrete molecule. Hence,
if liquid state or solid state is used,
added energy is required to break the intermolecular forces. Therefore, the gas state is used as the intermolecular forces between gas molecules are the weakest.
(D) Final set: This provides additional information.
 -- -- -- -- --Article written by Kwok YL 2009.
-- -- -- -- --Article written by Kwok YL 2009. Disclaimer and remarks:
- If you would like to use this source, kindly drop me a note by leaving behind a comment with your name and institution. I am all for sharing as the materials on this blog is actually meant for the education purpose of my students.
- This material is entirely written by the author and my sincere thanks will be given to anyone who is kind, generous and gracious to point out any errors.