Chemical bonding is an important topic, the interatomic bonds helps to account for many physical properties, energies evolved or required for chemical reactions and these bonds also account for the speed of reactions. The three interatomic bonds which we will discuss are
(1) Covalent bond,
(2) Ionic Bond and
(3) Metallic Bond.
(1a) Covalent Bond
Covalent bonds are formed when
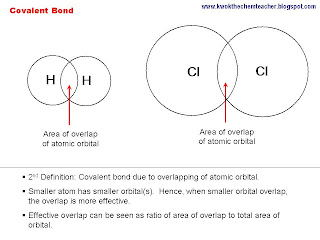
(A) there is electrostatic forces of attraction between shared electrons and the nuclei of the atom. However, in order for the two atoms to share electrons, the atomic orbtial of the constituting atoms must overlap with each other. Hence,
(B) covalent bonds are formed when
atomic orbitals overlap.

When two atomic orbital overlap, we obtained two molecular orbitals. (Since 1+1 = 2, we cannot just have one molecular orbital.) The two molecular orbitals are (i) Bonding orbital and (ii) Anti-bonding orbital. The illustration below provides greater details about the shape of (i) and (ii) and the relative energy level with respect to the atomic orbital.

However, if the the electrons are placed in the anti-bonding orbital, the bond breaks. (This is why in nucleophilic substitution, the nucleophile attacks the back of the C-X bond.)
P.S. The concept of Anti-bonding orbital is not in the GCE A level syllabus. The above picture is a basic illustration of Molecular Orbital Theory.
(1b) Multiple Covalent Bonds.
Using this diagram as our starting point, the number of electron pairs between two nuclei indicates the number of covalent bond between the two atoms. When there is one electron pair, this gives us a single bond. When there are two electron pairs between two nuclei, this gives us a double bond. Lastly, when there are three, it gives us a triple bond. Interestingly, we usually do not get more than 3 electron pairs between two nuclei. You can read more here.
(2) Ionic Bonds
Ionic bonds are formed because the electropostive atom has donated an electron to the electronegative atom. The former becomes a cation, while the latter becomes an anion. Electrostatic attraction between oppositely charged ions creates the ionic bond. Unlike covalent bonds, ionic bonds are not directional.

(3) Metallic Bonds
We are most commonly aware that metallic bond is formed because of electrostatic attraction between the array of cation and the sea of electrons. The illustration below depicts metallic bond and it helps in explaining the physical properties of metal.

However, when we boil liquid metal and form gaseous metal, do we obtain cations and electrons?
We don't. We actually obtain metal atoms, hence this is the limitation of the model which uses sea of electrons to describe metallic bonding. The more accurate illustration is given below but it is not in the A level syllabus. Do note that it is still a very simplified description.

Conclusion:
Electrostatic attraction results in chemical bonds to be formed, this attraction is a form of potential energy (PE). Due to the definition of PE, stronger the electrostatic attraction, the smaller the PE (note: numerical value gets larger).
As energy is conversed, formation of a bond (note: this refers to initially there is no bond between the two particles to a bond between the two particles.) results in a decrease in PE. Thus, there is a certain amount of energy lost when a chemical bond is formed which explains why bond formation is exothermic.
 -- -- -- -- --Article written by Kwok YL 2009.
-- -- -- -- --Article written by Kwok YL 2009. Disclaimer and remarks:
- If you would like to use this source, kindly drop me a note by leaving behind a comment with your name and institution. I am all for sharing as the materials on this blog is actually meant for the education purpose of my students.
- This material is entirely written by the author and my sincere thanks will be given to anyone who is kind, generous and gracious to point out any errors.