Acid chlorides (aka Acyl Chlorides), alkyl chlorides and aryl chlorides are halogen containing compounds. Generally, when we are asked to perform a chemical test to these three compounds, the aim of the chemical test is to see which of these compounds will be able to produce a AgCl precipitate. Hence, this test presupposes that a nucleophilic substitution must happen to release the Cl- so that it can be precipitated with a Ag+
When comparing alkyl chloride and aryl chloride. The chemical test steps consist of the following:
- NaOH, heat
- Cool
- Add HNO3
- Add AgNO3
The first step is the nucleophilic substitution where chloride ion leaves. Aryl chloride does not undergo nucleophilic substitution reaction.
While when comparing alkyl chloride and acid chloride. The chemical test is.
- Add AgNO3.
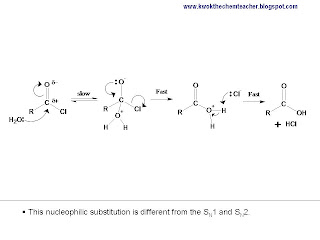
Acid chloride has a very electron deficient carbon, because it is attached to both the O and the Cl. That makes this carbon very willing for nucleophiles to attack it. Hence, in this chemical test, H2O is the nucleophile that causes a nucleophilic substitution to occur. Acid chloride is so susceptible to nucleophilic substitution that a weak nucleophile can be used (H2 vs OH-) and a milder condition is required (room temperature vs heat).
In addition, this post would also like to serve us a good entry which teaches us how to describe the nucleophilic substitution using acid chloride and a nucleophile. The mechanism is different from SN1 and SN2. The picture below describes the mechanism while the video below describes how to draw the mechanism.
-- -- -- -- --
Article written by Kwok YL 2010.
Disclaimer and remarks:
- If you would like to use this source, kindly drop me a note by leaving behind a comment with your name and institution. I am all for sharing as the materials on this blog is actually meant for the education purpose of my students.
- This material is entirely written by the author and my sincere thanks will be given to anyone who is kind, generous and gracious to point out any errors.
No comments:
Post a Comment