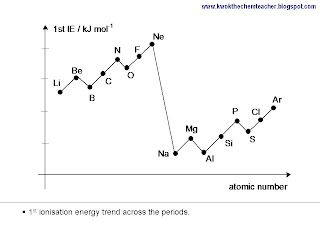
Generally, as we move across the period, the 1st ionisation energy increases. This is because despite an electron is added, the number of inner shells remains the same hence the shielding effect is relatively constant. While the nuclear charge increases. Therefore, the combination of these two reasons result in an overall increase in the 1st ionisation energy.
There are a couple of dips seen and these are exceptions to the trend. Generally, the dips are present because the causes for the dips are more significant than the effect due to increase in effective nuclear charge. The mind map below provides you with a structure which will explain the trend, including the exceptions.

In addition, there is another explanation to why the ionisation energy differs between period. We move from Period 2 to Period 3, the size of the atoms get larger. The added inner shell of electrons results in the valence shell of elements in Period 3 to be further away from the nucleus. Hence, electrostatic attraction between nucleus and valence shell becomes weaker, therefore 1st ionisation energy is smaller.
Article written by Kwok YL 2009.
- If you would like to use this source, kindly drop me a note by leaving behind a comment with your name and institution. I am all for sharing as the materials on this blog is actually meant for the education purpose of my students.
- This material is entirely written by the author and my sincere thanks will be given to anyone who is kind, generous and gracious to point out any errors.
No comments:
Post a Comment