Stereoisomerism is usually one of the most intriguing field to conceptual. It is relatively easier to conceptualise structural isomerism, which is basically the different ways of linking the atoms in a molecule. However, a pair of structures may have their atoms to be linked together via covalent bonds in a same fashion but the spacial arrangement of the atoms is different - this constitutes stereoisomerism.
There are two main types of stereoisomers. (i) Geometric Isomers and (ii) Optical isomers.
Optical Isomers
Optical isomers are an important discovery - since humans are sensitive to the type of optical isomer which we can absorb. Optical isomers are mirror image to each other. A structure contains a chiral carbon when there is no plane of symmetry intersecting that carbon (as seen below.)
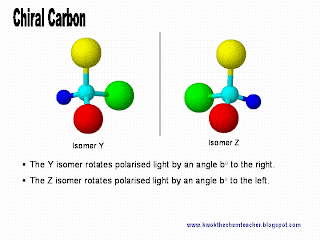 The only way we can distinguish the pair of enantiomer is to make use of polarised light. The direction which the polarised light rotates to will help to distinguish the pair of optical isomer.
The only way we can distinguish the pair of enantiomer is to make use of polarised light. The direction which the polarised light rotates to will help to distinguish the pair of optical isomer.

 The only way we can distinguish the pair of enantiomer is to make use of polarised light. The direction which the polarised light rotates to will help to distinguish the pair of optical isomer.
The only way we can distinguish the pair of enantiomer is to make use of polarised light. The direction which the polarised light rotates to will help to distinguish the pair of optical isomer.However, how can we be convinced that the pair of isomers, Y and Z, which are mirror images to each other, are unique too?
 Notice that by rotating of the isomer Z does not help you to superimpose it with isomer Y; they are still different from each other - you cannot get all four balls of one isomer to fit the other. You could try visualising rotation of isomer Z about another axis, and you will still obtain the same conclusion.
Notice that by rotating of the isomer Z does not help you to superimpose it with isomer Y; they are still different from each other - you cannot get all four balls of one isomer to fit the other. You could try visualising rotation of isomer Z about another axis, and you will still obtain the same conclusion.Hence, the easiest patten to observe a chiral compound is that when there are four groups that are distinct attached to a sp3 carbon atom will lead to optical active compounds. Although, correctly put, a sp3 carbon is chiral when there is no plane of symmetry intersecting it. (Do note that this is applicable to GCE A level syllabus, there is another chiral compound which has sp2 carbon.)
However, a word of caution. In the chemical reaction, sometimes both enantiomers are produced in equal amounts, therefore leads to a racemic mixture. This mixture contains both enantiomers and hence resulted in the polarise light to appear that it did not rotate, since the rotation caused by one enantiomer cancels out the rotation by the other.
Therefore, this gives a false sense that the product of the chemical reaction is achiral. Hence, chemists have been studying a field on assymmetric synthesis, so as to produce chiral compounds and not the racemic mixtures.
-- -- -- -- --
Article written by Kwok YL 2009.
Disclaimer and remarks:
- If you would like to use this source, kindly drop me a note by leaving behind a comment with your name and institution. I am all for sharing as the materials on this blog is actually meant for the education purpose of my students.
- This material is entirely written by the author and my sincere thanks will be given to anyone who is kind, generous and gracious to point out any errors.
2 comments:
Hi Mr Kwok,
In this entry it said that there is another compound with sp2 chiral carbon. Does it means that if a carbon atom is chiral it is not necessarily an sp3 carbon?
If so, when we determine whether a carbon atom is chiral we should look at whether a plane of symmetry exists instead?
Hi Dung,
In the A levels, we shall take it that it is sp3 only.
I am trying to search for the name of the molecule. Will let you know when I found it. But that is still not the highlight of your A level syllabus.
Post a Comment