Stereoisomerism is usually one of the most intriguing field to conceptual. It is relatively easier to conceptualise structural isomerism, which is basically the different ways of linking the atoms in a molecule. However, a pair of structures may have their atoms to be linked together via covalent bonds in a same fashion but the spacial arrangement of the atoms is different - this constitutes stereoisomerism.
There are two main types of stereoisomers. (i) Geometric Isomers and (ii) Optical isomers.
Geometric Isomers
The structures below show the same linkages, however, the arrangement of the atoms is different. The structure on the left has two H (denoted by the white sticks) on the same side of the double which is the cis structure, while the structure on the right has the H on opposite direction, which is the trans.
 It appears that it by twisting the double bond, one will be able to get from one structure to the other. Unfortunately, this twisting of about the double bond would cause the pi bond to be broken. This is energetically unfavourable at room temperature, hence both structures exist as distinct structures; not inter-changeable.
It appears that it by twisting the double bond, one will be able to get from one structure to the other. Unfortunately, this twisting of about the double bond would cause the pi bond to be broken. This is energetically unfavourable at room temperature, hence both structures exist as distinct structures; not inter-changeable.Hence, geometric isomers exist because of restricted rotation of a bond. This restricted rotation stems from the fact that a bond will be broken, which rotation is done.
Since, breaking of a covalent bond, pi or sigma, requires energy, such a change would require a high activation energy. - At room temperature, the inter-change between structures does not occur.
In the GCE 'A' Level syllabus, we basically discuss more about Geometric isomers due to presence of the double bond. In trying to twist the above molecule, we are rotating it about the axis where the sigma bond of the C=C lies.
 The twisting cause the p orbitals to not overlap from each other, which effectively breaks the strong pi bond - That is energetically unfavourable to occur at room temperature.
The twisting cause the p orbitals to not overlap from each other, which effectively breaks the strong pi bond - That is energetically unfavourable to occur at room temperature.Interesting, the single bonds are generally easy for rotation to occur. Therefore, straight chain molecules do not exhibit geometric isomers.
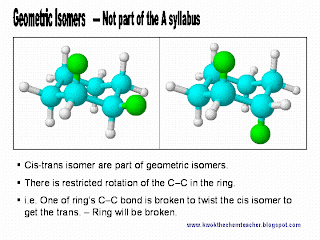
However, that does not implies that ring structures do not exhibit geometric isomers. In rotation of the C-C on difluorocyclohexane, to get both fluorine atoms to face the same general direction (e.g from both facing up to one facing up and one facing down), would actually cause the ring to snap. - (Note: Text is italic because this is not part of the GCE A level syllabus.)
 -- -- -- -- --
-- -- -- -- --Article written by Kwok YL 2009.
Disclaimer and remarks:
- If you would like to use this source, kindly drop me a note by leaving behind a comment with your name and institution. I am all for sharing as the materials on this blog is actually meant for the education purpose of my students.
- This material is entirely written by the author and my sincere thanks will be given to anyone who is kind, generous and gracious to point out any errors.
No comments:
Post a Comment