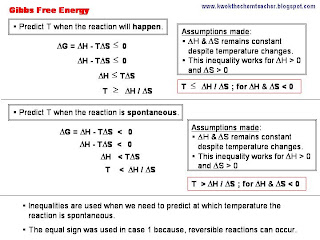
From the chart below, we can observe that there are certain chemical reactions whose change in Gibbs Free Energy change with temperature. Hence, we are able to perform mathematical calculations to predict at which temperatures the reaction will happen, become spontaneous and cease to occur.

(1) Predict the temperature for reactions to happen or become spontaneous.
The difference between the two terms is quite simple. Spontaneous chemical reaction implies that the change in Gibbs Free Energy is smaller than 0. Reaction whose change in Gibbs Free Energy is equals to 0 can happen; they are just reversible. Hence, for reactions to happen, the change in Gibbs Free Energy has to be smaller or equals to 0.
(2) Predict the temperature for reactions to become non-spontaneous.
In non-spontaneous reactions, we are implying that we need to determine temperatures where the reaction's change in Gibbs Free Energy becomes greater than 0.

(3) Units.
The temperature used in the formula to calculate change in Gibbs Free Energy is in Kelvin (K). While the unit for change in Enthalpy is in KJ mol-1 and the unit for change in Entropy is J mol-1K-1. Hence, you need to do the necessary conversion and exercise care when apply the formula.
-- -- -- -- --
Article written by Kwok YL 2009.
Disclaimer and remarks:
- If you would like to use this source, kindly drop me a note by leaving behind a comment with your name and institution. I am all for sharing as the materials on this blog is actually meant for the education purpose of my students.
- This material is entirely written by the author and my sincere thanks will be given to anyone who is kind, generous and gracious to point out any errors.
No comments:
Post a Comment