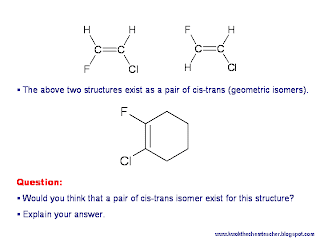
Hint: Some amount of visualisation is needed to answer the following question which is found in the picture below. - The answer is not that obvious.
Answers:

Comments:
(1) Congratulations to Yi-Min, Eugene, Joanna, Darrell and Brian. The five of you got the answer correct with a suitable explanation. The exact words are not the same, but the sense I have from your answer is that you understood the issue.
(2) Some answers actually mentioned that the trans cannot exist because the groups are difference hence they are geometrical isomers. That is highly incorrect. The molecule give to you actually has the pattern of the cis-trans isomer; since the left and right side of the ring (w.r.t the double bond) is the same.
(3) Some of you are confused between the idea of restricted rotation and existence of a particular stereoisomer. In sharing that the presence of a double bond, restricts the rotation hence cis and trans are mutually exclusive and not inter-changable does not exactly account for their existence.
Although, I must admit that it is a valid point when we are discussing about geometric isomerism about a double bond that is not on a ring, in this case it is not valid anymore.
(4) A word of encouragement: I think that the quality of answers, despite, it is not correct, is getting better. From your answers, I think many of you made valiant attempts to rationalise your choice and make your answer as coherent as you can. Kudos to that. =)
22 comments:
A pair of cis-trans isomers does not exist. In a theoretical trans-isomer, the ring is contorted such that the sp3 carbon atoms bonded to the sp2 carbon atoms are on opposite sides. The other 3 single C-C bonds not bonded to the sp2 carbons are not flexible enough to conform to this structure.
Therefore, only the cis-isomer exists, where the 2 sp3 carbon atoms attached to the 2 sp2 carbon atoms are on the same side.
For the molecule posted online, cis-trans isomers can exist for the structure of the molecule.
The molecule consists of a ring, with a fluorine and chlorine atom attached to two different carbon atoms next to each other in the ring structure.
For cis-trans isomerism to occur, the cis isomer must not be able to alternate to trans isomer without breaking of a bond. In this case, if in the first case, the fluorine atom is facing top, while the chlorine atom is facing downwards, the molecule is existing as a trans isomer. For both the chlorine and fluorine atoms to face in the same direction, either either upwards, or downwards, the entire ring must twist. In that case, not only would the double bond between the carbon atoms break, another C-C single bond would break.
Thus, the molecule cannot alternate between cis and trans isomers without at least a bond being broken. Therefore, the molecule exists as cis-trans isomers.
Exist.
The structure shown is a cis-isomer because there are 2 same substituent attached to the same side of the double bond. This substituent is the long single bond carbon chain. There are also at least 3 different substituents and a double bond exists which restricts rotation. Thus, since a cis-isomer exists, a trans also exists so a pair of cis-trans isomer exists.
No. Although the 2 groups attached to each sp2 carbon atom are different, and there is restricted rotation due to the presence of C=C double bond, the structure cannot have a trans isomer due to the ring structure. The ring structure cannot be on opposite sides of the C=C double bond as required of a trans isomer. Hence, cis-trans isomerism does not apply for this structure.
No. Only the cis isomer is present. Trans isomer is not possible due to the restricted rotation about the 6-member ring. By forming a trans isomer, the ring will snap as the C-C single bond will snap.
Yes, the structure has a pair of cis-trans isomer. By breaking the ring, F and C2H4 will be on one of the carbon and C2H4 and Cl will be on another carbon. the two C2H4 will be on opposite sides of the carbon-carbon double bond.
No, a pair of cis-trans isomer does not exist for this structure. In this structure, onle a cis-isomer is present as both the flourine and chlorine atom can only face in one general direction. The trans-isomer does not exist due to the restricted rotation about the 6-member ring. In order for both the flourine and chlorine atom to face in different general directions, the ring would have to be broken. As such, a cis-trans isomer does not exist for this structure.
Cis-trans isomerism can exist. The structure shown in the question is the cis isomer, because there are 2 CH2 substituent groups on the same side of the molecule. In order to obtain the trans, the molecule must be rotated about the double bond to get the two CH2 substituents to face different directions; one up and one down. This would cause the pi bond to break, and therefore, since there is restricted rotation about the double bond, cis-trans isomerism exists.
A pair of cis-trans isomers does not exist for this structure. The given sturcture has a Cl atom on one double-bonded carbon and a F atom on the other. The other functional groups attached onto each of the double-bonded carbons are identical because they are joined together as a ring structure. Hence, the given structure is now existing as a cis isomer. However, in order to get a trans isomer, the 2 identical group bonded to a pi-bonded carbon, will have to exist on the diagonally opposite ends of each other. In order to do this, the ring structure will have to be broken. By doing this, the functional groups will be changed, and in this case, will be completely different from the orginal structure. Hence a trans isomer does not exist.
The structure can be a cis isomer and a trans isomer but both cannot exist interchangeably. This is because in the process of transforming a cis isomer to a trans isomer(vice-versa), rotation of the double bond will cause the pi bond to be broken. However, the cis and trans isomers can exist independently because of the 2 H present.
A pair of cis-trans isomer does exist for the structure.
For cis-trans isomerism to exist, there must be restricted rotation about the pi-bond (presence of C=C double bond) and the two groups attached the each sp2 carbon must be different.
In this case, the two groups attached to one of the carbon in the C=C double bond are Cl and CH2CH2CH2CH2 (R), which are different. For the other carbon, the two groups attached to it are also different, which are F and R.
The cis-isomer will be the one with both Rs on the same side of the double bond while the trans-isomer will have both Rs on opposite sides of the double bond.
the molecule does not have a pair of cis-trans isomer. Firstly, the F and Cl are on the same plane and even if the 2 atom change places, it is possible to rotate the molecule to get back the original one. Secondly, it is impossible for the C=C to rotate such that a trans isomer is created.
A pair of cis-trans exist for this structure.
As in the diagram, it can be seen that the structure is a cis-isomer as the halides, chlorine and fluorine, is one the same side of the double bond while the cyclopentene occupies the other side.
For the trans-isomers to exist, it has to be viewed as the cyclopentene facing out of the plane while the pair of halides are facing into the plane. This would render one halide to face the bottom left and another halide top right with respect to the carbon double bond. The chlorine and fluorine would then be next to the organic compound on the respective sides as the cyclohexene is protruding across the double bond. Thus it can be seen that the halides and the organic compound are on the opposite sides of the double bond.
i think a pair of cis-trans isomers do exist for this structure. The cis isomer is as shown in the question where the chlorine and fluorine atom are on the same side of the bond. The trans isomer exist when fluroine and chlorine atoms are on opposite sides of the bond in which the remaining two bonds of the c=c double bonds links to form a cyclohexene ring. The trans isomer can be viewed on a 2d diagram as the cyclohexene ring portruding out of the plane of the paper while the halides are on the plane of the paper.
Yes, a pair of cis-trans isomer does exist for this structure as a double bond does exist between 2 carbon atoms.Furthermore, the structure cannot be twisted to form the other structure due to the restricted rotation about the C-C double bond.If twisted, the structure would then break.Thus, this structure contains a cis-trans isomer.
No, there are no cis-trans isomers for that structure.
The F and Cl atoms are covalently bonded to an sp2 carbon atom each, and each of the sp2 carbon atoms are bonded to 2 other carbon atoms.
No more bonds can be formed by the sp2 carbon atoms and the bonds around them are arranged in a trigonal planar. There is only one place where the F and Cl atoms can be with respect to the rest of the molecule. Even if the C=C bond is twisted and broken, no other molecule with the similar structural formula but different orientation of the F and Cl atoms will be formed.
Yes. A cis-trans isomer exsists in this structure. For a cist-trans isomer to exsist, there must be restricted rotation of a bond. Also, there must be presence of a double bond. In the above structure, it is a cyclic ring with one double bond. The geometric isomer is present at the double bond of the structure. The C=C is bonded to F atom and Cl atom. As they are on the same side, it exhitbits cis-isomerism. Also, because there is presence of double bond, there is restricted rotation about the double bond. Because large amount of energy is required to break the pi bond in the C=C, meaning higher activation energy, it is not energetically favourable at room temperature.
A pair of cis-trans isomer exists for this structure.
This is because, there is restricted rotation about the C=C double bond.
In order to get from one isomer to another (from cis to trans isomer), the pi bond in the C=C double bond would be broken when rotated.
In a cis-isomer, there are 2 same groups on the same side of the bond (which is C4H8)and in a trans-isomer; there are 2 different groups on opposite side of the bond (which is C4H8). This is because the given molecule exists in a monomer form. Therefore, cis-trans isomer can exist in the structure given.
Yes, I think that a pair of cis-trans isomer does exist.
This is because the twisting of about the C-C double bond to form cis-trans isomers would cause the pi bond to be broken. The cis isomer would exist as monomers: C(CH2)3F=CCl- and the trans isomer would be CFCl=C(CH2)3-. Hence both structures exist as distinct structures. Thus, cis-trans isomer does exist as there is restricted rotation of the bond and twisting breaks the pi bond.
(Mr Kwok, i dunno whether this will be a repeat post i'm posting, cos something happened just now while uploading this. i think the first post got through alr, but i'm uploading it again incase it really failed the first time. sorry.)
Yes, a pair of cis-trans isomer exists for this structure.
The monomer of this structure can form a cis-isomer when there are 2 same groups of (CH2)4 on the same side of the C=C bond. This can occur when one C atom is bonded to (CH2)4 and the other C atom of the same side can be bonded to the next (CH2)4 molecule from the next monomer. This will go on. F and Cl will be bonded to the C atoms on the opposite sides. Since there are 2 same groups [(CH2)4] on the same side of the C=C bond, cis isomerism exists.
To get a trans isomer, (CH2)4 will form on opposite sides, each on different C atoms. Similarly, one C atom will be bonded to (CH2)4 from the compound itself and the next C atom of the opposite side will be bonded to the (CH2)4 of the other monomer. This will give 2 different groups on opposite sides of the bond.
Since there is restricted rotation about the pi bond, cis isomer and trans isomer cannot exist interchangeably. Therefore, a geometric isomer defined as "breaking of a covalent bond required to get one isomer to another" does exist.
no, i do not think the molecule exhibits geometric isomerism.
this is due to the types of atoms attached to the ring structure.as a cis-trans isomer requires at least 2 atoms of the same kind to be on either side of the isomer:
X \ /Z Z\ /Z
C=C C=C
Z / \Y X/ \Y
cis trans
but the given molecule only has 1 of each the Cl and Fl atoms attached to the ring. although rotation about the double bond would cause the pi bond to be broken, it would not cause any isomer to be formed because the Cl and Fl atoms would still be attached in the same way.and rotation about the other C-C bonds would not cause any difference as they all have only 1 type of atom attached to them,H atoms.
Hence the molecule does not exhibit stereoisomerism.
Yes, cis-trans isomers do exists for the structure. Due to the presence of the double bond (pi bond) and the ring, rotation of the structure,would effectively break the strong pi bond - That is energetically unfavourable to occur at room temperature. This makes it possible for F and Cl to face the same direction and in another instance to face different directions (e.g from both facing up to one facing up and one facing down) hence cis-trans isomers exists.
Post a Comment