In the study of the reactions involving carbonyls, one of the most important reactions is the nucleophilic addition reaction (You may like to refer to the definitions of reactions by clicking here.). This is similar to the reactions involving alkylhalides as both requires nucleophiles and electron-deficient carbon atoms. However, carbonyl will show an addition across a double (this time is the C=O), while the alkyl halides shows a replacement (aka substitution.)
The most significant nucleophilic addition reaction which we study in detail, is that between a carbonyl molecule and hydrogen cynanide. This reaction is studied in sufficient detail such that we are able to identify the most suitable conditions for the reaction. In addition, we were able to understand how sometimes NaOH or NaCN were used in trace amounts to boost this reaction.
Racemic mixtures can be formed in the reaction between the carbonyl and HCN. This is because the carbonyl is planar and the nucleophile can attack either side of the plane (of carbonyl) in equal probability. In addition, the carbonyl must first be asymmetrical. If the carbonyl is symmetrical, no racemic mixture is formed.
The following diagrams depicts the illustration of mechanism of the nucleophilic addition reaction when NaOH or NaCN is being used.
(i)When NaCN was used - Regeneration of CN-:
The most significant nucleophilic addition reaction which we study in detail, is that between a carbonyl molecule and hydrogen cynanide. This reaction is studied in sufficient detail such that we are able to identify the most suitable conditions for the reaction. In addition, we were able to understand how sometimes NaOH or NaCN were used in trace amounts to boost this reaction.
Racemic mixtures can be formed in the reaction between the carbonyl and HCN. This is because the carbonyl is planar and the nucleophile can attack either side of the plane (of carbonyl) in equal probability. In addition, the carbonyl must first be asymmetrical. If the carbonyl is symmetrical, no racemic mixture is formed.
The following diagrams depicts the illustration of mechanism of the nucleophilic addition reaction when NaOH or NaCN is being used.
(i)When NaCN was used - Regeneration of CN-:
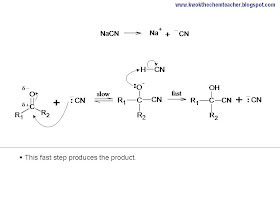 (ii)When NaOH was used - Regeneration of OH-
(ii)When NaOH was used - Regeneration of OH-
While, the following video helps to demonstrate how the mechanism is drawn.
-- -- -- -- --
Article written by Kwok YL 2009.
Disclaimer and remarks:
- If you would like to use this source, kindly drop me a note by leaving behind a comment with your name and institution. I am all for sharing as the materials on this blog is actually meant for the education purpose of my students.
- This material is entirely written by the author and my sincere thanks will be given to anyone who is kind, generous and gracious to point out any errors.
No comments:
Post a Comment