The basic idea of the structure of an atom is that it contains protons and neutrons which make up the nucleus and electrons which circulate around the nucleus. In basic courses of chemistry, one learns that electrons are filled in shells and each shell encircles the nucleus, hence giving an atomic structure which is shown below.
 Hence, if we examine the energy required to remove successive electron of an atom we will obtain a linear graph as depicted below. The increasing graph shows that it becomes increasing difficult to remove an electron from a particle which is increasing positive.
Hence, if we examine the energy required to remove successive electron of an atom we will obtain a linear graph as depicted below. The increasing graph shows that it becomes increasing difficult to remove an electron from a particle which is increasing positive. The graph will be coherent to the above model of a structure of an atom. That is because the electrons in a particular shell receives the same electrostatic attraction from the nucleus. (P.S. energy to remove an electron is known as ionisation energy).
The graph will be coherent to the above model of a structure of an atom. That is because the electrons in a particular shell receives the same electrostatic attraction from the nucleus. (P.S. energy to remove an electron is known as ionisation energy).However, the actual graph showing the ionisation energies of the atom is shown below. The graph shows that the removal of the 11th and 12th electron is more difficult than the earlier electrons and that using the reason "removal of an electron from a particle which is getting more positive" is insufficient. Hence, this implies that the 11th and 12th electron are closer to the nucleus than the 13th to the 18th electron.
 Hence, the concept of orbital and subshell is conceived. The orbital is the area where the electrons are found. In the A level syllabus, we focus on three types of orbitals, (i) s, (ii) p and (iii) d. Their illustration is shown below and each orbital can only occupy a maximum of two electrons.
Hence, the concept of orbital and subshell is conceived. The orbital is the area where the electrons are found. In the A level syllabus, we focus on three types of orbitals, (i) s, (ii) p and (iii) d. Their illustration is shown below and each orbital can only occupy a maximum of two electrons.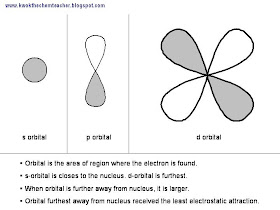 Therefore, there is the idea of subshell; where each subshell contains a number of orbtial. The s-subshell contains just one s orbital, while the p-subshell contains three p orbital and the d-subshell contains five d orbtial.
Therefore, there is the idea of subshell; where each subshell contains a number of orbtial. The s-subshell contains just one s orbital, while the p-subshell contains three p orbital and the d-subshell contains five d orbtial. This idea of atomic structure is the correct interpretation of the atomic structure and it allows for us to account for the ionisation energies of the atom.
This idea of atomic structure is the correct interpretation of the atomic structure and it allows for us to account for the ionisation energies of the atom.Article written by Kwok YL 2009.
Disclaimer and remarks:
- If you would like to use this source, kindly drop me a note by leaving behind a comment with your name and institution. I am all for sharing as the materials on this blog is actually meant for the education purpose of my students.
- This material is entirely written by the author and my sincere thanks will be given to anyone who is kind, generous and gracious to point out any errors.
No comments:
Post a Comment