- The reactant particles must collide.
- The reactant particles must collide in the right orientation.
- The reactant particles must possess a minimum kinetic energy.
(1) Particles must collide.
To understand why the reactant particles must collide, I think it is important to realise that in a chemical reaction, there will generally be chemical bonds (and usually it is covalent bonds) that are broken and new ones formed.
Covalent bonds are intramolecular bonds which are formed because the atomic orbitals of the atoms overlap, allowing for the sharing of electrons between the two atoms and these shared electrons can be attracted by the nuclei of the atoms - therefore the two atoms are held to each other; giving rise to a covalent bond.
Hence, when two particles collide, it allows for the rearrangement of the orbital which will overlap with each other and hence resulting in an rearrangement of the chemical bonds, hence a reaction occurring. This can occur because during a collision, orbital of one reactant particle can overlap with the orbital of another reactant particle.
(2) Collision must be of the correct orientation.
The following illustrations describe why collision must be in the right orientation.
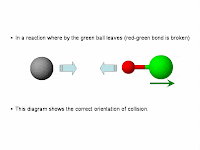
In this orientation, the black ball's orbital can overlap with the relevant orbital on the red ball hence assisting in the rearrangement of chemical bonds.To understand why the reactant particles must collide, I think it is important to realise that in a chemical reaction, there will generally be chemical bonds (and usually it is covalent bonds) that are broken and new ones formed.
Covalent bonds are intramolecular bonds which are formed because the atomic orbitals of the atoms overlap, allowing for the sharing of electrons between the two atoms and these shared electrons can be attracted by the nuclei of the atoms - therefore the two atoms are held to each other; giving rise to a covalent bond.
Hence, when two particles collide, it allows for the rearrangement of the orbital which will overlap with each other and hence resulting in an rearrangement of the chemical bonds, hence a reaction occurring. This can occur because during a collision, orbital of one reactant particle can overlap with the orbital of another reactant particle.
(2) Collision must be of the correct orientation.
The following illustrations describe why collision must be in the right orientation.

(Note 1: The relevant orbital is always in opposite line of direction where the red-green bond is. It is in that orientation so as to facilitate the acceptance of electrons to break the red-green bond. - You can learn more from Note 2.)
(Note 2: In order to understand the relevant orbital on the red ball, one needs to know the concept of bonding and anti-bonding orbital. It is not in A level syllabus)

In this orientation, the black ball's orbital is not able to overlap with the relevant orbital on the red ball hence no reaction occurs.
(3) Reactant particles must possess the minimum activation energy.
From the above illustration, the red-green bond is broken. Since, a chemical bond is broken hence energy is needed to overcome the attraction between shared electrons and nuclei of the red and green balls. Thus, this energy comes from the kinetic energy of the reactant particles.
Hence, it the reactant particles moves too slowly, they do not possess sufficient energy to break the bond. Hence, no reaction can take place.
Concluding thoughts:
Are there limitations in this theory? Can you think of any? Post them in the comments page!
-- -- -- -- --
Disclaimer and remarks:
- If you would like to use this source, kindly drop me a note by leaving behind a comment with your name and institution. I am all for sharing as the materials on this blog is actually meant for the education purpose of my students.
- This material is entirely written by the author and my sincere thanks will be given to anyone who is kind, generous and gracious to point out any errors.
No comments:
Post a Comment