As we move across Period 4, moving from K to Cu, we observe the graph below which shows the trend of how atomic radius changes with an equal increase in proton and a corresponding increase in electron.
1. Decreasing size of atom across the period (general trend).
Like in every period we have observed. The general trend we observe is that there is a general decrease in the size of the atomic radius. This trend is similar to what we see in the red box.
To account for this trend, the basic idea is that the effect of the increased nuclear attraction due to the increase in number of protons is more significant than the effect of shielding due to the adding of electrons. Normally, across the period, we add electrons in the same shell and the shielding due to this addition is relatively insignificant.
Hence, the shielding of the outermost electrons (4s electrons) is due to the inner shell. However, in the case of the transition metals, it is the addition of an electron in the 3d subshell. Although, the 3d subshell is in the inner shell, it is relatively weak in shielding as compared to a quantum shell (which is what K and Ca experience). Therefore, it is not surprising that the transition metals are smaller than K or Ca.
2. Relatively constant atomic radius observed amongst transition metals.
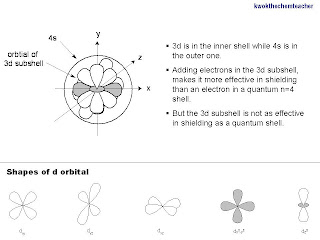
From the diagram below, you can see that the 3d subshell is an inner shell which can shield the 4s electrons.
Hence, despite there is an increase number of protons which results in an increased nuclear attraction, the addition of an electron in a 3d subshell shields the 4s electrons better than when an electron is added to a subshell which is found in shell with the quantum number 4. This happens because the 3d electrons are in an inner shell.
Therefore, the effect of increasing nuclear charge will somewhat be neutralised by the increase in shielding effect due to the addition of an electron to the 3d subshell. This results in the effective nuclear charge to remain relatively constant as we move across the period. Therefore, the size of atoms to be approximately the same.
In addition, the gradual filling of the 3d subshell, improves its ability to shield the 4s electrons from the nucleus.
Moreover, this diagram which shows how the 3d subshell is formed. It actually forms an asymmetrical sphere that shields the 4s electrons. A quantum shell forms a more symmetrical sphere that shields the outer shell. Hence, this is why the 3d subshell remains relatively poor in shielding.
3. Application.
The application of the above explanation will also help to account the 1st ionisation energy trend across the entire period 4 as well as the trend seen for just the transition metals.
-- -- -- -- --
Article written by Kwok YL 2010.
Disclaimer and remarks:
- If you would like to use this source, kindly drop me a note by leaving behind a comment with your name and institution. I am all for sharing as the materials on this blog is actually meant for the education purpose of my students.
- This material is entirely written by the author and my sincere thanks will be given to anyone who is kind, generous and gracious to point out any errors.

No comments:
Post a Comment