Primary and secondary alcohols are susceptible to oxidation reactions using K2Cr2O7/H+. While tertiary alcohol are resistant to oxidation reactions. Secondary alcohols when oxidised, produces ketones. However primary alcohols can be oxidised to two different products; an aldehyde or a carboxylic acid. The type of product produced is dependent on the conditions of the reaction.
From the above illustration, it can be seen that if we heat the primary alcohol (example shows ethanol) with K2Cr2O7/H+ under reflux conditions we will get our carboxylic acid. While if we heat the primary alcohol with K2Cr2O7/H+ using a distillation setup we will be able to obtain the aldehyde.
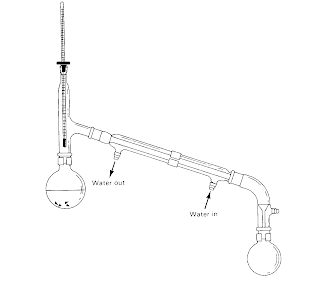
The reflux apparatus is shown above. A condenser is attached about the round-bottom flask which contains the reaction mixture. Cold water is passed through the condenser from the bottom and the warmer water is removed from the top. In this set-up, the reactants/products will undergo a cycle of evaporation and condensation.
This setup is extremely purposeful for the formation of carboxylic acid when primary alcohols are oxidised. This is because the primary alcohols will first be oxidised to the aldehyde and because the reflux setup tracks the product, the aldehyde is able to be subsequently oxidised by K2Cr2O7/H+ to produce the carboxylic acid.
Hence, in order to produce just the aldehyde, a distillation setup (shown below) is used. This setup allows the aldehyde to be distilled out of the reaction mixture. This is because the aldehyde has a lower boiling point than ethanol and water(water is the solvent) as in only has permanent dipole-permanent dipole interaction as its most predominant Inter-molecular forces. This interaction is weaker than the hydrogen bonding which both ethanol and water has.
Hence, the distillation process allows the aldehyde to escape, preventing it from being oxidised by the oxidising agent. This entry serves as an avenue to explain the difference in the oxidation reaction of the primary alcohol.
-- -- -- -- --Article written by Kwok YL 2010.
Disclaimer and remarks:
- If you would like to use this source, kindly drop me a note by leaving behind a comment with your name and institution. I am all for sharing as the materials on this blog is actually meant for the education purpose of my students.
- This material is entirely written by the author and my sincere thanks will be given to anyone who is kind, generous and gracious to point out any errors.



No comments:
Post a Comment