On the other hand, alkyl groups when attached to the benzene ring, donate electron density towards the ring and hence making the benzene ring more reactive toward electrophiles.
Hence, is the electron donating property of alkyl groups stabilising or destabilising?
Well, the answer is lies in the effect that the electron donating property causes. In the case of carbocations (and radicals), the alkyl groups donates their electron density towards the electron deficient carbon.
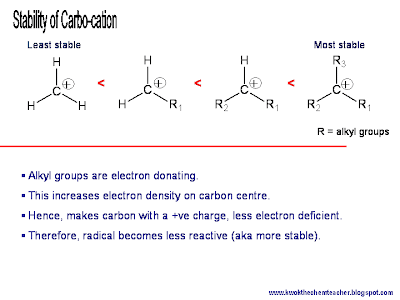
Hence, by donating the electron density, the alkyl group is no longer neutral in charge but rather carry some partial positive charge; inevitably sharing the positive charge (or the extent of electron deficiency) with the carbon that has the positive charge.
However, in the case of the methylbenzene, the alkyl groups donate electron density to the electron ring. This makes the pi electron ring richer in electron density and hence more susceptible to electrophilic attacks.
It is timely to remind ourselves that in the electrophilic substitution mechanism, the slow step is the reaction between the benzene ring and the electrophilc.
Hence, seeing the effect caused by the alkyl groups will lead us to making the correct choice to whether they stabilises or destabilises.
-- -- -- -- --
Article written by Kwok YL 2010.
Disclaimer and remarks:
- If you would like to use this source, kindly drop me a note by leaving behind a comment with your name and institution. I am all for sharing as the materials on this blog is actually meant for the education purpose of my students.
- This material is entirely written by the author and my sincere thanks will be given to anyone who is kind, generous and gracious to point out any errors.
No comments:
Post a Comment