Previously we have discussed about Bimolecular Nucleophilic Substitution, also known as SN2. In this mechanism, the rate of the reaction is dependent on the concentration of the alkyl halide and the concentration of the nucleophile. Hence, this also implies that both alkyl halide and the nucleophile are involved in the slow step of the reaction.
However, sometimes the rate of nucleophilic substitution reaction between an alkyl halide and nucleophile (note: aryl halides are STILL unable to undergo this reaction. Click here to find out why.) can be dependent on the concentration of the alkyl halide and independent on the concentration of the nucleophile.
Therefore, the above paragraph suggested that there is an alternative mechanism (or you could call it reaction route) for the nucleophilic substitution reaction. This reaction is called unimolecular nucleophilic substitution, SN1.
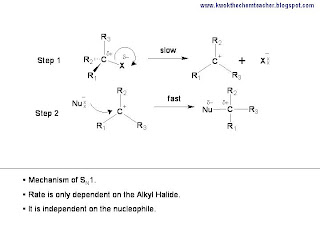
There are two steps in this mechansim. The first step is the breaking of the C-X bond to generate the carbocation.
However, sometimes the rate of nucleophilic substitution reaction between an alkyl halide and nucleophile (note: aryl halides are STILL unable to undergo this reaction. Click here to find out why.) can be dependent on the concentration of the alkyl halide and independent on the concentration of the nucleophile.
Therefore, the above paragraph suggested that there is an alternative mechanism (or you could call it reaction route) for the nucleophilic substitution reaction. This reaction is called unimolecular nucleophilic substitution, SN1.
There are two steps in this mechansim. The first step is the breaking of the C-X bond to generate the carbocation.

While the second step, which is the fast step, is where the nucleophile is attracted to the carbocation.

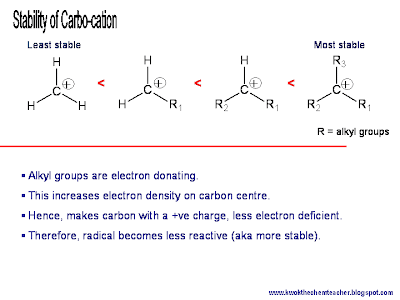
The rationale to why SN1 is possible is because there are reasons to support the formation of a carbocation. If a stable carbocation can be formed, the SN1 mechanism would be likely to occur. (Click here to read how the carbocation can be stablised.)
As such, generally 2o alkyl halide, 3o alkyl halide and phenyl-halo-methane (e.g. a benzene ring with a -CH2X substituent), will support a SN1 mechanism because it can form a stable carbocation. How C6H5CH2+ carbocation is stablise by the phenyl group follows the same principle as this.
In conclusion, if you need to describe the mechanism of Unimolecular Nucleohilic Substitution, SN1, both the fast and slow steps must be drawn. Hence, the following illustration shows the requirements needed.
As such, generally 2o alkyl halide, 3o alkyl halide and phenyl-halo-methane (e.g. a benzene ring with a -CH2X substituent), will support a SN1 mechanism because it can form a stable carbocation. How C6H5CH2+ carbocation is stablise by the phenyl group follows the same principle as this.
In conclusion, if you need to describe the mechanism of Unimolecular Nucleohilic Substitution, SN1, both the fast and slow steps must be drawn. Hence, the following illustration shows the requirements needed.
The video below provides the steps and some basic explanation to SN1 mechanism.
-- -- -- -- --
Article written by Kwok YL 2009. Updated in Feb 2010.
Disclaimer and remarks:
- If you would like to use this source, kindly drop me a note by leaving behind a comment with your name and institution. I am all for sharing as the materials on this blog is actually meant for the education purpose of my students.
- This material is entirely written by the author and my sincere thanks will be given to anyone who is kind, generous and gracious to point out any errors.