With the introduction of entropy; which indicates the distribution of particles in a system does not help us to predict whether a reaction occurs or not. This is because we do observe reactions which show an increase in disorder (positive entropy change) and reactions which show a decrease in disorder (negative entropy change) happen.
However, we do observe that certain reactions, which has a certain enthalpy change and certain entropy change, can take place at certain temperature but will be unable to do so at other temperatures.
This observation allows for the introduction of Gibbs free energy. The change in Gibbs free energy accounts for the change in enthalpy and the change in entropy. It is this term, when negative in value, tells us that the reaction will be spontaneous, while when it is positive, tells us that the reaction is non-spontaneous. This term is a direct application of 2nd Law of Thermodynamics.
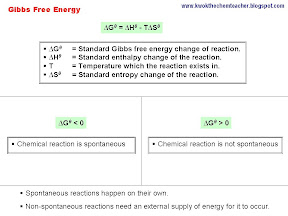 |
In addition, it is vital that we are able to appreciate that by changing temperature, it can sometimes cause a chemical reaction which was initially spontaneous to become non-spontaneous as we can see from the diagram below.

It is good to take note that when temperature change, when we apply the above formula we assume that the enthalpy change and entropy change remains the same despite there is a change in temperature. (Or at least, that the change in entropy and change in enthalpy due to temperature cancels each other out.)
However, the change in temperature may lead to the substances (in the chemical equation) to be in a different phase at the new temperature as compared to the old one (An example is shown below). This phase change results in a significant change in the entropy and hence we cannot assume that the change in entropy remains constant at a different temperature.
While at the "A" level Chemistry syllabus, we do assume that enthalpy change remain the same despite temperature change. This is because the assumption is that the enthalpy change of the reaction is affected solely by the chemical bonds present in the substances and that these bonds remain of the same strength even at a higher temperature. - (Although, I must add that this assumption is used to simplify the calculation of enthalpy change.)
In conclusion, reaction that is spontaneous will happen on its own in nature, while reactions that are not spontaneous will need an external supply of energy so that it can take place - for example photosynthesis is one such process, thus planets make use of light energy to enable this reaction to take place.
-- -- -- -- --
Article written by Kwok YL 2009.
- If you would like to use this source, kindly drop me a note by leaving behind a comment with your name and institution. I am all for sharing as the materials on this blog is actually meant for the education purpose of my students.
- This material is entirely written by the author and my sincere thanks will be given to anyone who is kind, generous and gracious to point out any errors.
No comments:
Post a Comment