(A) Strength of Covalent Bond:
Definition 1: Covalent bond is formed when there is electrostatic attraction between shared electrons and the nuclei of the two atoms.

When an atom is large, this will result that its valence electrons to be found further for the nucleus. In addition, the atom only make use of its valence electrons to form the covalent bond. Hence, when two larger atoms form a covalent bond with each other, their shared electrons will be significantly further away for the nucleus. Hence, the electrostatic attraction is weaker.
Definition 2: Covalent bond is formed when there is an overlapping between two atomic orbitals.
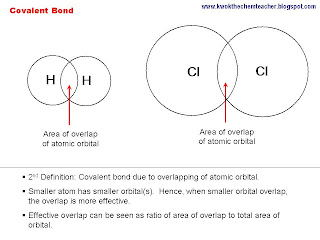
The atoms make use of their atomic orbital, where the valence electrons occupies, to overlap with each other and hence the a covalent bond is formed. The larger the atom, the less effective the overlap. Hence, the weaker the covalent bond.
Explanation - Strength of Covalent Bonds.
In addition, because of the definition of potential energy, this result in the covalent bond of H-H to be stronger and yet lower in potential energy than the Cl-Cl bond. This is succintly illustrated by the diagram below. Hence, lower potential energy implies greater electrostatic attraction between shared electrons and nuclei.
Lastly, bond energy refers to the amount of energy required to break a covalent bond. A larger bond energy implies that more energy is required to break a covalent. Hence, small atoms form strong covalent bond because the distance between shared electrons and nuclei is small. Therefore, greater electrostatic attraction, which results in more energy required to overcome this attraction, thus small atoms form bonds with large bond energies.
 Other factors that affect strength of covalent bonds include: A polar covalent bond strengths the bond between two atoms. In addition, when two small atoms each having a lone pair of electrons, there is repulsion between the electron pairs hence, weakens the covalent bond.
Other factors that affect strength of covalent bonds include: A polar covalent bond strengths the bond between two atoms. In addition, when two small atoms each having a lone pair of electrons, there is repulsion between the electron pairs hence, weakens the covalent bond.In summary, using the other concepts you see in covalent bonding, the following flow-chart will be useful when we try to explain the different strengths of covalent bonds.

-- -- -- -- --
Article written by Kwok YL 2009 (updated May 2009).
- If you would like to use this source, kindly drop me a note by leaving behind a comment with your name and institution. I am all for sharing as the materials on this blog is actually meant for the education purpose of my students.
- This material is entirely written by the author and my sincere thanks will be given to anyone who is kind, generous and gracious to point out any errors.
No comments:
Post a Comment