Alkenes react with halogen molecule via an Electrophilic additon mechanism. First, we need to define an electrophile. An electrophile can be defined as a species which is electron deficient which has the ability to accept a pair of electrons.
Therefore, in the mechansim there must be an electrophile that starts the reaction. The halogen molecule in the reaction between alkene and halogen is the electrophile.
In addition, there are three possible medium where the halogen molecule may be used. In this example, I will be using the reaction between ethene and bromine to illustrate the electrophilic addition mechanism.
When ethene reacts with Br2 which is dissolved in CCl4. The following picture describes the mechanism.
Therefore, in the mechansim there must be an electrophile that starts the reaction. The halogen molecule in the reaction between alkene and halogen is the electrophile.
In addition, there are three possible medium where the halogen molecule may be used. In this example, I will be using the reaction between ethene and bromine to illustrate the electrophilic addition mechanism.
When ethene reacts with Br2 which is dissolved in CCl4. The following picture describes the mechanism.

When ethene reacts with Br2 which is dissolved in H2O, Br2 is still the electrophile that reacts with the alkene. The following picture describes the mechanism.
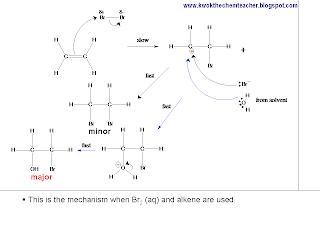
However, when ethene reacts with Br2 which is dissolved in NaCl solution, the following picture describes the mechanism. Again, Br2 is the electrophile which reacts with the alkene
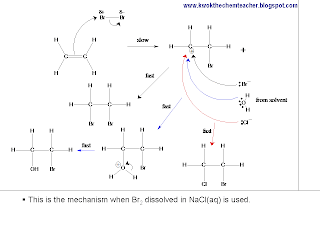
In conclusion, the following video helps to explain "how to describe electrophilic addition reaction" questions.
-- -- -- -- --
Article written by Kwok YL 2009.
Disclaimer and remarks:
- If you would like to use this source, kindly drop me a note by leaving behind a comment with your name and institution. I am all for sharing as the materials on this blog is actually meant for the education purpose of my students.
- This material is entirely written by the author and my sincere thanks will be given to anyone who is kind, generous and gracious to point out any errors.
No comments:
Post a Comment